Surviving HIV
November 9, 2010 § 3 Comments
![]() Untreated, HIV is normally a death sentence. But not quite always. A small number of people infected with HIV can survive for decades without symptoms. They’re called “elite controllers”, and — although the fact that they’re healthy makes them hard to identify with certainty — they’re thought to comprise less than 1% of the infected population.
Untreated, HIV is normally a death sentence. But not quite always. A small number of people infected with HIV can survive for decades without symptoms. They’re called “elite controllers”, and — although the fact that they’re healthy makes them hard to identify with certainty — they’re thought to comprise less than 1% of the infected population.
Elite controllers, as the name suggests, control the replication of HIV much better than a normal infected person. Although they’re definitely infected, they have very low (to undetectable) amounts of virus circulating in their bloodstream. They are therefore much less likely to pass on the infection, and they maintain perfectly normal levels of CD4 cells. For these few lucky individuals, HIV may be merely an inconvenience.
What makes them special? A genome-wide association study, performed as a result of an impressive collaborative effort (the list of authors is longer than the paper), has come up with a simple and satisfying answer: the genes most clearly associated with being an elite controller are essentially all variants of MHC class I, and identifying the subtypes of MHC class I that are over-represented in the elite controller population makes it possible to pinpoint a handful of amino acids in the peptide-binding groove as important for protection.
Let me unpack that a little for non-immunologists. The immune system has a difficult and important problem: it needs to protect you against pathogens that are constantly evolving. A large and important part of the immune system focuses on parts of pathogens that are hard for the pathogen to change, but this is not enough. Vertebrates have evolved a second layer of protection, the adaptive immune system, which has the job of looking for anything foreign. The way it does this is elegant, though not without problems — essentially, it learns not to attack anything “normal”, and then attacks everything else.
The core of the system is a pair of protein complexes called MHC class I and class II. Both have the job of collecting bits of proteins — which could be foreign when you’re infected with something, but most often aren’t — and making them visible to other parts of the immune system. Class I has the job of monitoring the interior of the cell, and is therefore especially important for viral infections; class II monitors the exterior of the cell. Both class I and class II have a structure resembling a bear trap at one end of the molecule, made of two alpha-helices positioned on top of a floor constructed of beta-sheets and displayed on the outer membrane of the cell. The bear trap captures peptides derived from the proteins in the environment (which may or may not be foreign) and holds them out for inspection. In the case of MHC class I, the inspector is a cytotoxic T cell; if it sees something it doesn’t like in the jaws of the trap, it will attack and kill the cell carrying the MHC/foreign peptide complex. Of course this “steel trap” analogy has flaws: it’s probably better to think about the MHC molecule folding around the peptide to create a new hybrid protein structure that is partly determined by MHC sequence and partly determined by peptide sequence. The hybrid has a unique shape, and this is what the cytotoxic T cell recognizes.
Both class I and class II MHC genes are highly polymorphic: there are lots of different alleles in the population, each of which can bind a different characteristic subset of the peptides that might be produced by a pathogen. And so people who carry different MHC subtypes may be more or less efficient at fighting off specific diseases. (They all bind different subsets of the peptides produced by your normal proteins, too, hence the association of different MHC subtypes with autoimmune diseases such as rheumatoid arthritis). The differences in peptide binding are, not surprisingly, mostly due to residues within the bear trap, either on the jaws or on the floor.
Which brings us back to the findings of this paper. The International HIV Controllers study identified ~300 significant associations of SNPs in a comparison of elite controllers with other HIV-infected patients. In the European subset of their patient population, this turned out to be due to just four independent markers of association. All of them turned out to be in the region of the genome that encodes the three different gene families of class I MHC (HLA-A, B and C).
Although most of the patients in the study had not been tested for specific MHC subtypes, the authors were able to confidently infer which MHC subtypes they carry using SNP/MHC associations from a different study. As I have written about before, the strongest association for non-progressors of European descent was with the MHC subtype HLA-B57, but this study now identifies several more subtypes as protective, and others as associated with progression.
Having identified the most comprehensive list yet of MHC subtypes that are good and bad for HIV control, the authors did some sequence-gazing to try to identify specific amino acids that might be responsible for the effect. Although there is a great deal of variation between the amino acid sequences of these MHC class I subtypes (MHC being the most polymorphic region of the human genome), they were able to identify six amino acid locations as independent markers of non-progression: 97, 67, 62 and 63 in HLA-B, 77 in HLA-A, and 304 in HLA-C. Position 70 in HLA-B is also highly associated with non-progression, but it’s highly linked to positions 67 and 97. The identities of the amino acids at these three positions — 67, 70 and 97 — show stronger associations to controller status than any one classical MHC class I subtype sequence.
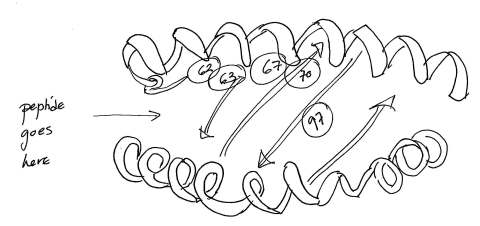 I’ve shown some of the important amino acids on a sketch of the MHC class I bear trap. It looks pretty clear that these amino acid locations are going to influence both which peptides are bound by the MHC, and how exactly they’re presented to the cytotoxic T cell. Position 97, right on the floor of the peptide-binding groove, makes the single largest difference: with a valine residue at that position, you are far more likely to be a controller than if you have an arginine or a serine. Alas, of the six possible amino acids you could have at that position in your own personal HLA-B molecules, arginine (bad) is the most common (51%), while valine (good) is quite rare (3%). Probably this is part of the explanation of why elite controllers are so infrequent.
I’ve shown some of the important amino acids on a sketch of the MHC class I bear trap. It looks pretty clear that these amino acid locations are going to influence both which peptides are bound by the MHC, and how exactly they’re presented to the cytotoxic T cell. Position 97, right on the floor of the peptide-binding groove, makes the single largest difference: with a valine residue at that position, you are far more likely to be a controller than if you have an arginine or a serine. Alas, of the six possible amino acids you could have at that position in your own personal HLA-B molecules, arginine (bad) is the most common (51%), while valine (good) is quite rare (3%). Probably this is part of the explanation of why elite controllers are so infrequent.
You can also, in this story, begin to see why MHC genes are so polymorphic. Random changes in MHC, especially in the peptide-binding groove, have a real chance of being immediately helpful to you if they happen to enhance your immune reaction to a passing virus. Since the diseases you encounter are likely to have quite different “preferences” for MHC sequence, the selection pressure they exert is unlikely to lead to an overwhelming advantage for one type of MHC over another; hence, diversity. The immune system is truly a wondrous thing, with many beautiful (though complicated) lessons for us all.
The International HIV Controllers Study (2010). The Major Genetic Determinants of HIV-1 Control Affect HLA Class I Peptide Presentation Science : 10.1126/science.1195271
Interesting!
I am wondering would be possible to make a new type of vaccine based on the published results?
It’s an interesting question. I’m not sure how relevant this finding is to the problem of preventing HIV infection in the first place. Once you’re infected, perhaps these results might help design a “therapeutic vaccine” to slow progression. But, any such intervention would presumably need to be tailored to specific MHC class I subtypes. At least, that’s my guess.
[…] infected with HIV-1, but have very low amounts of virus circulating in the blood. The results of a genome-wide association study identify a handful of amino acids in the peptide-binding groove of the MHC class I molecule as […]