Looking for synergy in unexpected places
July 15, 2011 § 1 Comment
I talk a lot about drug-resistant bacteria and why we should worry about their inexorable rise — the most recent example of which is chronicled here. Now I want to offer you another thing to worry about: drug-resistant fungi. It’s the same general problem — when you use a drug that inhibits the growth of some organism, and you use it a lot, that organism has a real incentive to evolve around the drug. The special worry with fungi, though, is that we never had a huge array of useful drugs in the first place. The best broad-spectrum antifungals are the azole derivatives, such as fluconazole; these inhibit an essential enzyme that is the product of the gene ERG11. But — same old story — they’re gradually losing their effectiveness.
Why are effective, broad-spectrum antifungals so rare? The problem is not so much that fungi are hard to kill, it’s that they’re hard to kill without killing us as well. Fungi are eukaryotes, and the pathways they use to thrive and survive are awfully similar to the analogous pathways in us. Even fluconazole suffers from this problem: the ERG11 gene encodes a cytochrome P450 enzyme, and we humans have many similar enzymes; cross-reaction of fluconazole with the human enzymes causes significant toxicity. The fact that fungi and humans have so much in common has made it hard to identify single agents that reliably kill (or inhibit the growth of) one, while sparing the other.
A new study (Spitzer et al. 2011. Cross-species discovery of syncretic drug combinations that potentiate the antifungal fluconazole, Mol. Syst. Biol. 7 499) now offers hope that combinations of drugs will do better. Spitzer et al. started with the observation that although there are only 1100 genes in the yeast Saccharomyces cerevisiae (biologists’ favorite model fungus) that are essential under normal lab conditions, many more genes can become essential under other conditions. In particular, if you knock out one non-essential gene, you often find that you can no longer knock out certain other non-essential genes without killing the yeast. This is called synthetic lethality. Roy Kishony likes to use the following analogy to explain it: suppose you put an eyepatch over your right eye. This may make you look as if you’re auditioning for Pirates of the Caribbean 5 (or are we up to 6 now?), but it doesn’t completely prevent you from seeing. The same is true if you put the patch over your left eye. It’s only if you wear two patches, one over each eye, that you get the “synthetic lethal” effect on your vision. Similarly, knocking out two non-essential genes — or, in this case, inhibiting their products with drugs — may have a lethal effect even though targeting just one of the two genes doesn’t do much.
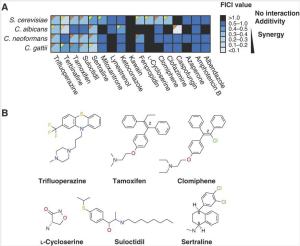
To expand the universe of useful antifungal drugs, Spitzer et al. wanted to look for synthetic lethal combinations that involve drugs not currently used as antifungals. They took a library of bioactive drugs, including a number of clinically approved drugs that are no longer covered by patents, and screened them in combination with fluconazole at a concentration where fluconazole isn’t effective on its own. They used four different fungi: our old friend S. cerevisiae, and the human pathogens Candida albicans, Cryptococcus neoformans, and Cryptococcus gattii. Almost 150 compounds — over 10% of the library — showed activity against one or more of the fungi, including examples of some surprising drug classes including antidepressants, antibiotics, and antipsychotics. (I guess even fungi get depressed.) These drugs were not active against fungi on their own, but in combination with fluconazole they had an effect. You could call them conditional antifungals.
The authors chose 12 of their newly discovered conditional antifungals for more detailed followup. Several of these show true synergy with fluconazole when studied in concentration matrix assays (the effect of the mixed drugs is greater than you would expect if you add up the effects of the individual drugs). Two are “broad spectrum”: the antidepressant sertraline (better known as Zoloft®) and the antipsychotic trifluoperazine each act synergistically with fluconazole on all four of the fungi tested. Other drugs have specific effects on one or two fungi. Encouragingly, many of these drug combinations actually are lethal — fluconazole alone only stops fungi from growing, but the combinations marked with orange triangles in the figure are fungicidal, not fungistatic.
Now, the thing about synergistic lethality is that it can also come about through drug-gene interactions. A gene knockout can remove the activity of one non-essential gene, and a drug can remove the activity of another; if the two genes interact, then so does the drug-gene pair. Screening for such interactions has turned out to be useful in tracking down which of the (often varied) biochemical effects of a drug are important for its function. Using this approach, the authors were able to identify genes that, when knocked out, make yeast more sensitive to the actions of the drugs. Of the six newly discovered conditional antifungals they tested, five behaved similarly: loss of certain genes involved in the trafficking of membrane vesicles makes the effects of these drugs much worse. In cell biological assays, these compounds + fluconazole also messed up the localization of membrane-associated dyes. The authors call these drugs the “membrane active” set. The other compound, L-cycloserine, showed specific interactions with genes encoding an enzyme involved in the synthesis of sphingolipids. Those of you who are interested in using drugs to perturb membrane trafficking should stop reading this blog post and go read the paper; there’s lots more there.
Could we have predicted the interactions between these drugs and fluconazole? Maybe. In a retrospective analysis, Spitzer et al. took the profiles for drug-gene interactions they had generated in the yeast genetic screens, and combined them with a gene-gene interaction map assembled from literature data. Then they looked at whether the set of genes that showed increased fluconazole sensitivity also showed stronger-than-expected links with the set of genes that interacted with the 5 “membrane active” drugs. It seems that they do, suggesting that this approach might allow us to pick out potentially synergistic drug pairs using computational analysis, if we have the data sets and pathway maps required for such an analysis. In fact, the authors identified some additional potential conditional antifungals using literature data on drug-gene interactions, and these drugs turned out to be modest to weak hits in their original screening data set, which is pretty encouraging. Of course, it’s hard to tell at this stage how powerful these predictions will be; and we don’t have these databases for all the pathogens we might want to discover treatments for. But, one must start somewhere.
The fact that many of these drug combinations are species-specific is worth commenting on in itself. It’s not surprising, but it’s important. An effective antifungal drug combination must kill one eukaryote (the fungus) but spare another (you). Nearly all the basic pathways we rely on for life are conserved between fungi and man, hence the effectiveness of studying yeast as a way of gaining insight into human biology. The logic of many pathways is the same, and often the most important individual proteins are well conserved as well. But of course there are differences, otherwise there would be only one species of eukaryote. We’re hoping here to target the differences — most often, differences of regulation and wiring, rather than dramatic differences in protein function — and so it’s not surprising that we see selectivity in many cases. One might imagine that by targeting two points in a pathway using a drug combination, you could achieve a more selective effect than is usually possible with just one drug.
An unanswered question in this excellent paper is that of how human cells react to these combinations. These are combinations of approved drugs, and one would therefore assume that they’re likely to be safe: but in combination they have a new effect on fungi and so their effect on human cells may also have changed. One hopeful piece of data comes from a test of the fluconazole/sertraline combination in a model infection in vivo, using infected wax moth caterpillars. The caterpillars showed much better survival in response to combination treatment than when treated with either drug alone; so the drug combinations are at minimum not universally toxic.
This is a long post, even for me, and I should stop; but just a couple of final thoughts, because I think this paper represents an exciting new trend. Combination therapy is increasingly important in medical practice, but the tools we have to discover effective combinations are almost laughably primitive. We take drugs that are effective individually (and individually approved for use in the clinic), check to see whether their toxicities obviously overlap, and if not we try them out as combinations. Combinations like the ones discovered here, where the activity of the second drug only becomes evident in the presence of the first drug, can’t be developed using this method. So research (like the work in this paper) that helps to make it easier to discover or design rational combinations of drugs has the potential to open up whole new areas of therapeutic approaches — as well as being intrinsically cool, of course.
Spitzer M, Griffiths E, Blakely KM, Wildenhain J, Ejim L, Rossi L, De Pascale G, Curak J, Brown E, Tyers M, & Wright GD (2011). Cross-species discovery of syncretic drug combinations that potentiate the antifungal fluconazole. Molecular systems biology, 7 PMID: 21694716
Really intesting post. It makes me think about how little we know about drug-gene interactions (not only drug-drug interactions) and how ironic this is, because of the role of drugs in human life.